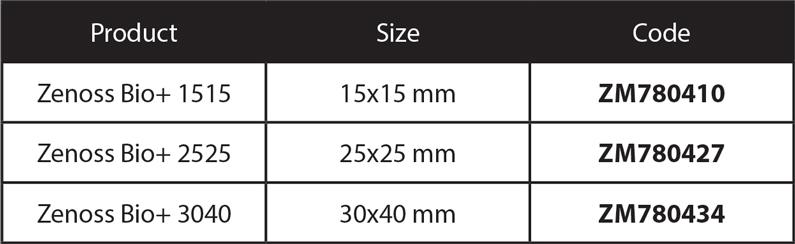
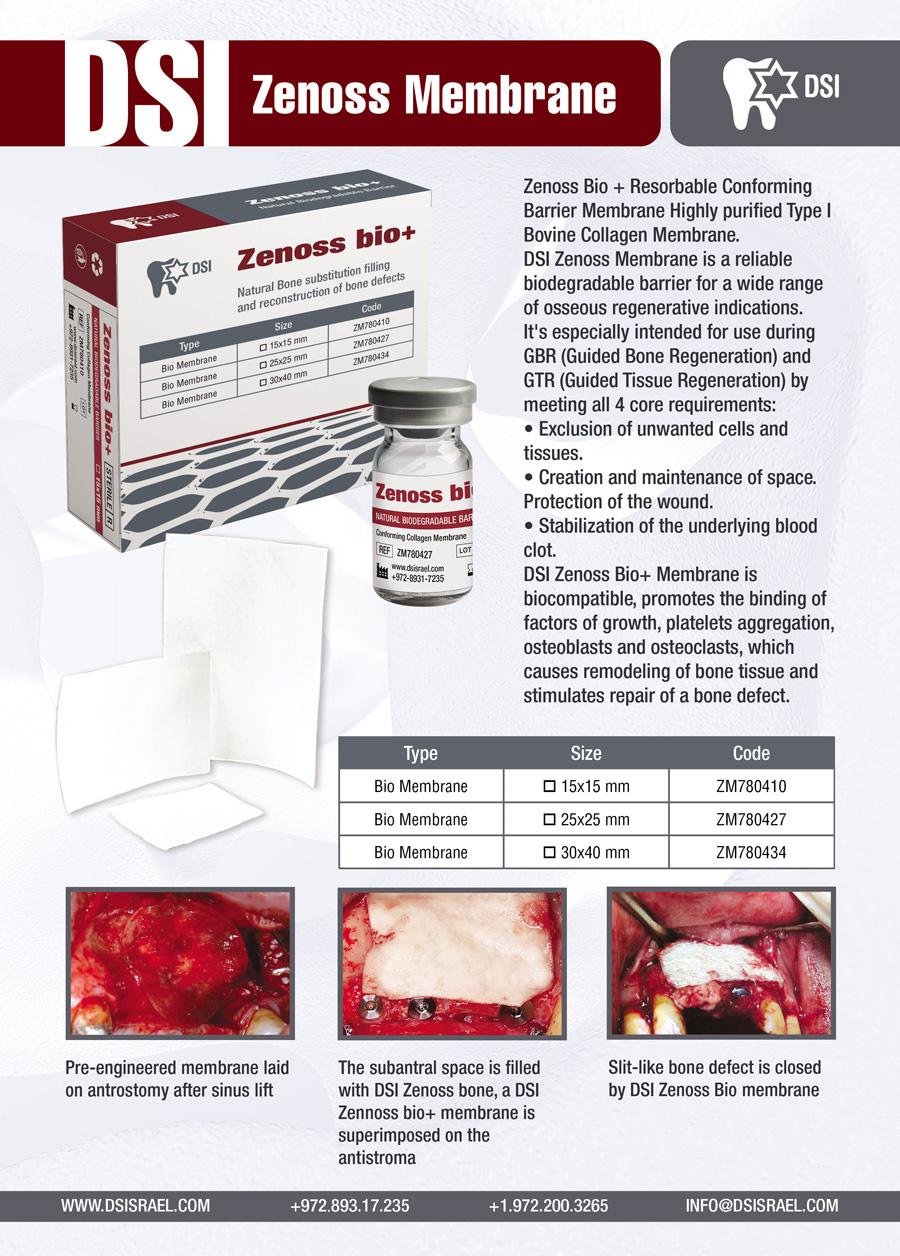
Zenoss Bio Membrane
Zenoss Bio + Resorbable Conforming Barrier Membrane is a Highly purified Type I Bovine Collagen Membrane. DSI Zenoss Membrane is a reliable biodegradable barrier for a wide range of osseous regenerative indications. It’s especially intended for use during GBR (Guided Bone Regeneration) and GTR (Guided Tissue Regeneration) by meeting all 4 core requirements:
• Exclusion of unwanted cells and tissues.
• Creation and maintenance of space.
• Protection of the wound.
• Stabilization of the underlying blood clot.

DSI Zenoss Bio+ Membrane is biocompatible, promotes the binding of factors of growth, platelets aggregation, osteoblasts and osteoclasts, which causes remodeling of bone tissue and stimulates repair of a bone defect. A suture pull-out strength of DSI Zenoss Membrane is significantly higher due to its unique fibrillar matrix structure. A longer degradation profile (16- 24 weeks) suits for the healing time required in most GBR procedures. It is resistant to a large variety of bacteria, which is of vital importance in a wound dressing. It helps to keep the wound sterile, because of its natural ability to fight infection. DSI Zenoss Bio+ is engineered from highly purified type I collagen from young steers tissues, thus providing such key factors as an increased resorption period, fast hydration, excellent tensile strength, easy shaping and trimming, to ensure optimal bone regeneration. Clinicians can be confident that DSI Syntoss Bio+ will serve as a perfect solution at GTR and GBR cases such as:
• Extraction Sockets.
• Site preparation for the implant.
• Bony defects and Alveolar ridge preservation.
• Peri-implant bone defects around implants.
• Sinus floor augmentation over the lateral window and under the Schneiderian membrane tears.