DSI Internal Hex ImplantsRBM Premium Implant
DSI recently developed DSI RBM Premium Line Implant – a latest generation high level improved implants, which combine all the late researches and developments in the industry. DSI RBM Premium Line of implants is designed to improve and speed up the healing process and the growth of the tissues adjacent to the implant surface. Macroscopic and especially microscopic qualities of the implant surface play an important role in the implant adaptability. DSI RBM Premium Line has a spiral thread design with twin cutting grooves and a redirecting ability. The coronal micro-treads designed to increase bone-implant contact and provide long-term crestal area stability. Recommended for two-stage surgical procedures, but suitable for the single-stage placement as well. DSI RBM implants are packaged in our ISO Class 7 Clean Room. The implants are supplied in a sealed vial made of two parts. The special package allows the implant to stay intact. This prevents exposure to any foreign materials that may contaminate the ultra-pure surface. Designed for maximum comfort and enhanced ergonomics.
At DSI, our objective is to provide safe and high precision dental products and services to the clients.
This is why each product undergoes the strictest quality control measures. We ensure that a high percentage of samples are taken from every batch. Each sample passes a very strict set of tests of cleanliness and conformity thus ensuring minimal rejections afterward. All of our products adhere to the highest international standards. All DSI Implants implants are made of titanium alloy Ti-6Al-4V ELI, in accordance with ASTM-F136-02.
RBM Premium Line Implant Features:
• Fixture model: Tapered.
• Tread design: Spiral, self-tapping, progressive for increased BIC and improved primary stability.
• Connection: 2.42 Internal Hex, Zimmer-SV compatible .
• Platform switching ability: One abutment platform for all diameters 3.5-5.0.
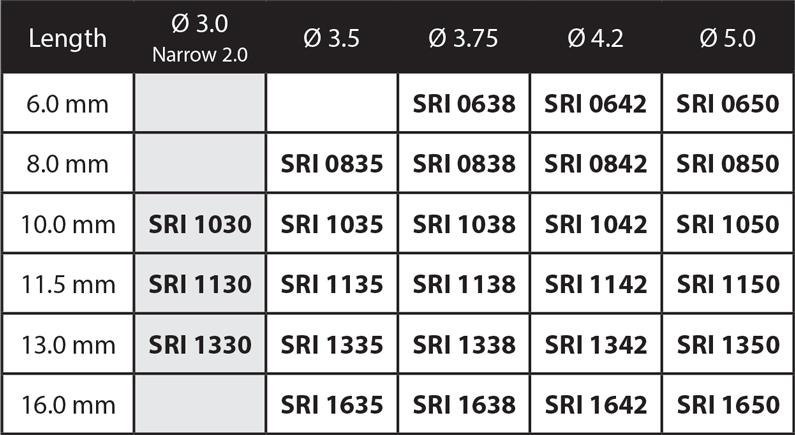
• Slim ridge Narrow 3.0 platform option available.
• Coronal part design: Micro rings -minimize peri-implant bone loss.
• Apical design: Rounded apex for safe and non-invasive placement.
• Increased fixture-abutment contact area.
• Material: Ti-6Al-4V ELI, in accordance with ASTM-F136-02.
• RBM Surface Treatment.
Resorbable Blast Media (RBM) Treatment:
The surface treatment is based on high-speed particle blasting, using the resorbable bioceramics – Beta-tricalcium phosphate (β-TCP) material. The surface is cleaned and etched from calcium particles with the organic acid of low concentration. This doesn’t change the titanium surface pattern while achieving the uniform surface and homogenous pore diameter and larger BIC. β-TCP is the resorbable material, often used for synthetic bone grafting. It’s completely dissolved and replaced by the new bone formations. Cleaner Implant surface – eliminates the risk of leaving contamination debris after blasting.

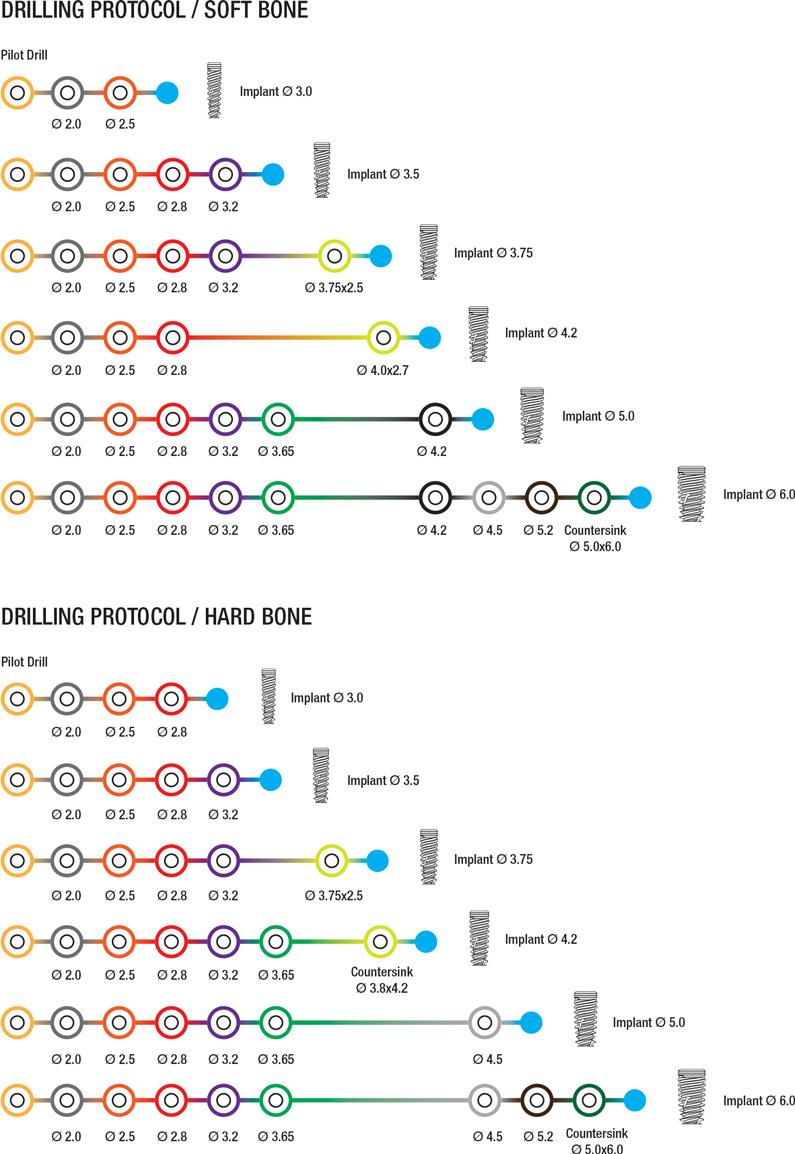
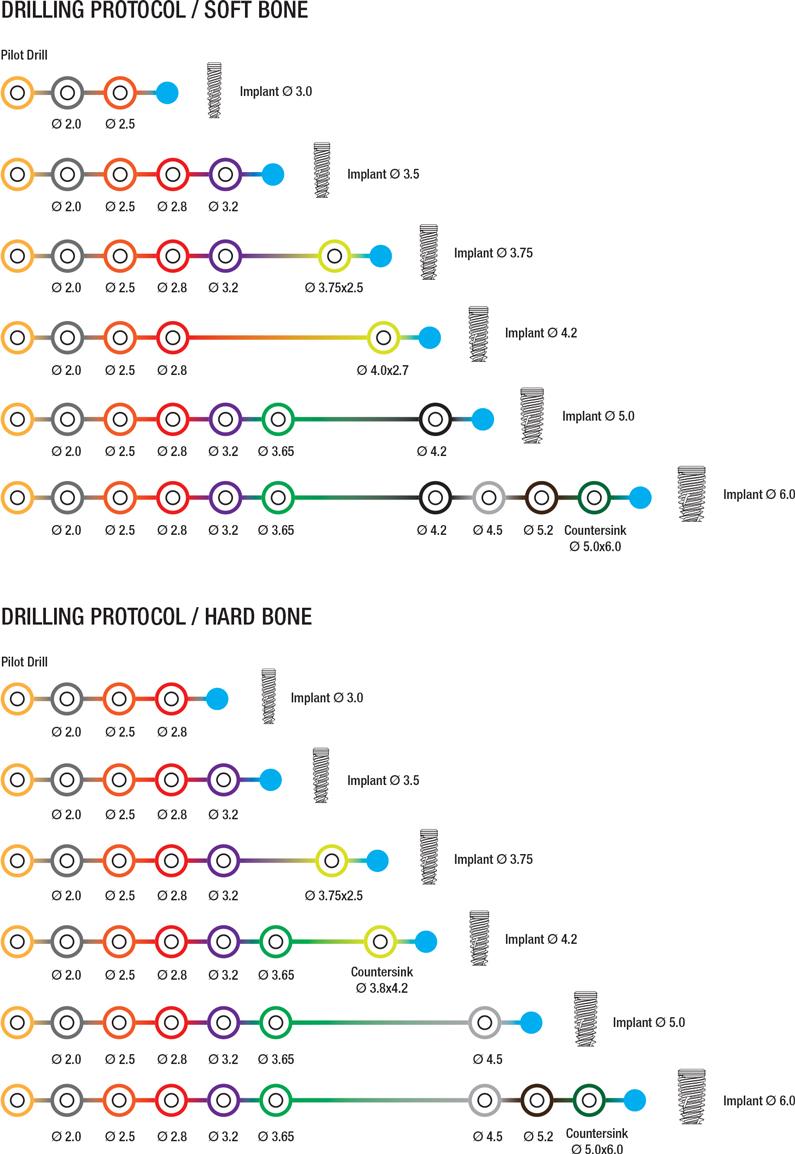
RBM Premium Line Implant drilling protocol: