CharacteristicsHeterologous origin biomaterial made of 600-1000 µm or 1000-2000 µm pre-hydrated collagenated cortico-cancellous granules, properly mixed with collagen gel. Thus, it is possible both skipping the hydration phase and decreasing the risk of accidental exposure of the material to pathogens during manipulation and grafting phases; furthermore, the syringe is flexible and ideal to simplify grafting in the receiving site.
The granules are endowed with characteristics very similar to human mineral bone, and can be used as an alternative to autologous bone.
Their natural micro-porous consistency facilitates new bone tissue formation(1) in defect sites and accelerates the regeneration process.
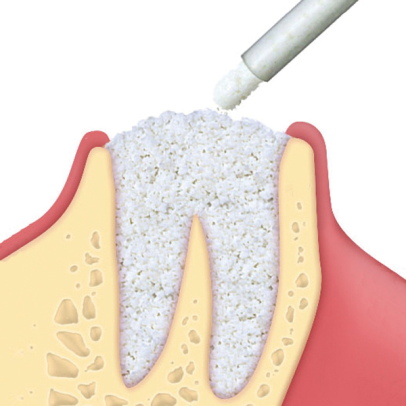
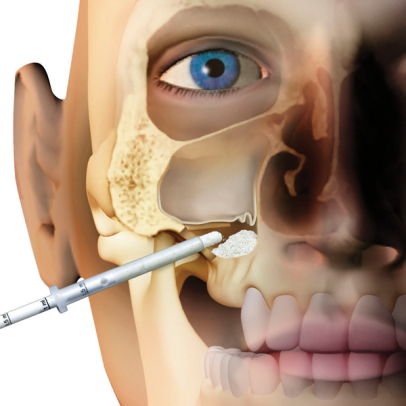
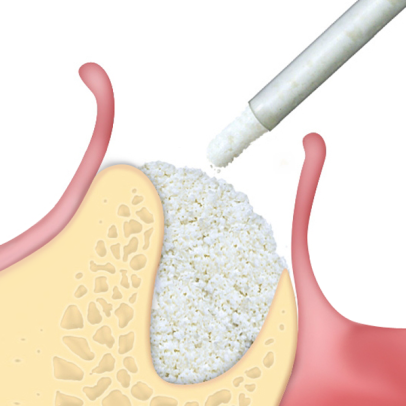
Gradually resorbable(2,3), it preserves the original graft shape and volume (osteoconductive property)(4,5). Moreover, thanks to its collagen content, the product facilitates blood clotting and the subsequent invasion of repairing and regenerative cells.Handlingmp3® is available in ready-to-use syringes and can be easily grafted avoiding the hydration and manipulation phases. After adapting the material to the defect shape, it is necessary to remove non-stable residues before proceeding to soft tissue suture. It is recommended to always compact mp3® after grafting to achieve optimal stabilization.Clinical informationmp3® is a pre-hydrated cortico-cancellous bone mix with 10% collagen gel. It has been developed with this innovative biotechnology and is a “ready-to-use” product. mp3® is commonly used for lateral access maxillary sinus lift(1,6), always in association with Evolution membranes, to cover the antrostomy: the mp3® syringe can be directly applied into the bony window without having to mix the mp3® granules with saline. Due to its collagen gel content, mp3® allows an excellent graft stability while its hydrophilia guarantees quick blood absorption and therefore the necessary graft vascularization. mp3® has also been successfully used in combination with Evolution membranes for alveolar ridge preservation(3,7,8): the application of this biomaterial limits the alveolar ridge width and height reduction that would naturally occur with spontaneous healing, preserving thus the alveolar ridge volume and allowing a correct second stage implant placement.
mp3® has been documented for horizontal augmentation (two wall defects) in combination with autogenous bone blocks or with OsteoBiol® Lamina(9,10): its cortico-cancellous composition allows a progressive resorption of osteoclastic type, and in parallel a similar rate of new bone formation(2). These unique properties allow a very good graft volume preservation(11), a healthy new bony tissue and ultimately, a successful implant rehabilitation.
- Tissue origin :
Cortico-cancellous heterologous bone mix - Tissue collagen :
Preserved plus an additional 10% collagen gel - Physical form :
Pre-hydrated granules and collagen gel - Composition :
90% granulated mix, 10% collagen gel - Granulometry :
600-1000 µm
1000-2000 µm - Re-entry time :
About 5 months - Packaging :
Syringe: 0.5 cc, 1.0 cc, 3 x 0.25 cc, 3 x 0.5 cc, 3 x 1.0 cc
Wide tip syringe: 2.0 cc - Product codes :
600-1000 µm
A3095FS | 1 Syringe | 0.5 cc | Porcine
A3095FE | 1 Syringe | 0.5 cc | Equine
A3005FS | 1 Syringe | 1.0 cc | Porcine
A3005FE | 1 Syringe | 1.0 cc | Equine
A3075FS | 3 Syringes | 3×0.25 cc | Porcine
A3015FS | 3 Syringes | 3×0.5 cc | Porcine
A3015FE | 3 Syringes | 3×0.5 cc | Equine
A3030FS | 3 Syringes | 3×1.0 cc | Porcine
A3030FE | 3 Syringes | 3×1.0 cc | Equine
A3010FS | 1 Wide tip syringe | 2.0 cc | Porcine
A3010FE | 1 Wide tip syringe | 2.0 cc | Equine
1000-2000 µm
A3210FS | 1 Wide tip syringe | 2.0 cc | Porcine
A3210FE | 1 Wide tip syringe | 2.0 cc | Equine